Where research meets real-world demand
The state-of-the-art research at The Parc focuses on five key domains: Solid state chemistry, Preformulation and solid state analysis, Drug design and process, Biopharmacy, and Pre-clinical in vivo testing. All research is carried out using the sophisticated technological support of Zentiva as well as top notch academic resources of our partners. What makes the venture exciting for the students is mainly the fact that more often than not they see the results of their research project implemented in real world.
-
Solid state chemistry
Taylor made active pharmaceutical ingredient (API) morphology and its impact on formulation process

Morphology and size of API crystals is crucial for its dissolution but also for its process ability during formulation process. In this project we are combining various methods for preparation of API crystals of various forms. These are consequently tested using small scale apparatuses developed to mimic conditions during formulation process to investigate impact of processing conditions on the properties of original API particles. In particular, we are interested whether original crystals survive such formulation process and if not, what would be the size and morphology of formed fragments. In addition, we are also testing their properties during dissolution of tablets containing such fragments. This information is essential when selecting most suitable formulation process for given initial API crystals or assessment of the robustness of formulation process.
Solid form screening, In-silico cocrystal prediction
Many currently discovered drugs have very low solubility in water, which results in their low bioavailability. The way to increase drug solubility is to use different drug solid forms including polymorphs, salts, co-crystals or accommodation of drug in polymeric matrix in amorphous state. The main goal of such a project is to develop methodologies for faster screening of new drug solid forms. This is done by combination of experimental investigation and modelling of inter-molecular interactions. To understand the significance of various interactions, we are using solution of crystal structure of prepared solid measured by X-ray diffraction combined with other techniques, e.g. Raman spectroscopy, differential scanning calorimetry, nuclear magnetic resonance etc.
Co-crystal development
One possibility of improving drug solubility and therefore also its bioavailability it to prepare drugs in the forms of co-crystals. Other parameter which influence the drug dissolution rate is the size and morphology of crystals. In this work, I am investigating an impact of drug partner molecule (i.e. coformer) in a co-crystal and process conditions on the chemical and physical properties of drug co-crystals. This includes also optimization and scale up of the crystallization process to prepare sufficient amount of desired co-crystals for consequent preformulation development.
Description and monitoring of solid dosage form disintegration and API precipitation
Currently, tablets are the most effective oral drug dosage forms. To develop proper dosage form it is essential to know the impact of various excipients and formulation process on the tablet disintegration and drug release rate. The aim of my work is to develop methods for characterization of tablet disintegration during dissolution tests to determine the rate of drug release, as well as structure, size and morphology of formed fragments. Furthermore, we would also investigate capability of various excipients to prevent precipitation of already dissolved drugs and thus improving their bioavailability.
Development of methods for characterization of oral dispersible films
Oral dispersible films represent relatively new approach for oral drug dosage. This, however, brings many questions about how to properly characterize their properties including drug dissolution rate, drug homogeneity as well as properties of such a film. The main goal of my project is to develop and consequently apply methods for thin polymer films characterization, which can be later on used in the quality control.
-
Preformulation and solid state analysis
Tablet disintegration

To develop a new methodology for drug product characterization, disintegration project has been opened. The goal of the project is deep understanding and description of tablet disintegration. Our approach is based on retrospective engineering with a focus on primary materials, standard unit operations and manufacturing steps. We are proceeding from a simple binary tablet to a more complex real formulation prepared by different technology (direct mixing, dry/wet granulation). Various non-standard (magnetic resonance imaging, Raman mapping, focused beam reflectance measurement, contact angle measurement, texture analysis) as well as common USP methods are used for tablet characterization during disintegration in order to describe the disintegration mechanism(s).
Chemical stability of APIs and drug products
The project is focused on development of forced degradation study system for API at different conditions (temperature, humidity, light, pH and oxidative conditions). Furthermore, real formulations containing API and excipients are studied and the obtained data is used for stability prediction based on comparison of experimental and in-silico data.
Hansen solubility parameters of pharmaceuticals
APIs and excipients are characterized by surface properties using Hansen solubility parameters (HSM). Different techniques - such as inverse gas chromatography and solubility method - are used for HSM determination and the values obtained from different techniques are simultaneously compared. The effect of crystal structure and particle morphology will be also studied.
Thermokinetic characterization and thermal stability of amorphous drugs
The project is focused on development of a new methodology for easy and efficient determination of thermal stability of amorphous drugs. This methodology will be based on the extrapolated predictions of crystallization kinetics calculated by coupling the differential scanning calorimetry measurements with state-of-the-art kinetic software. In addition to the macroscopic monitoring of the crystallization process, we also plan to utilize microscopic techniques in order to determine the influence of sub-Tg glass-crystal growth on the long-term drug stability/storage.
-
Drug design and process
Formulation using porous particles

We have developed mesoporous silica materials with a special pore structure to enable ultra-fast drug loading and drug release in order to achieve unparalleled control over the dissolution kinetics of poorly soluble drugs. We explore formulation processes for hot-melt sorption and solvent impregnation to porous carriers, as well as their scale-up. We employ advanced process analytical tools and computer simulations to understand the process across length-scales.
Computer simulation of fluid bed coating
We are developing mathematical models and computer simulation tools for accurately predicting the effect of operating parameters and material properties on the quality and structure of multi-layer pellets produced in a fluidised process by the spray coating of drug nanoparticle suspensions.
3D printing of medicines
We are exploring additive manufacturing technologies in the context of pharmaceutical formulation and processing, as tools to create desired structures with defined distribution of active ingredients and customised dissolution properties.
Formulation with liquid marbles
Liquid marbles are spherical droplets coated by a thin layer of powder. Our research aims at using these unique structures for advanced formulation of poorly soluble drugs. We explore processes and methods for the reproducible and scalable manufacturing of liquid marbles and for the rational design of their composition so as to achieve the desired end-use properties.
-
Biopharmacy
Predicting bioavailability enhancement through smart dissolution design
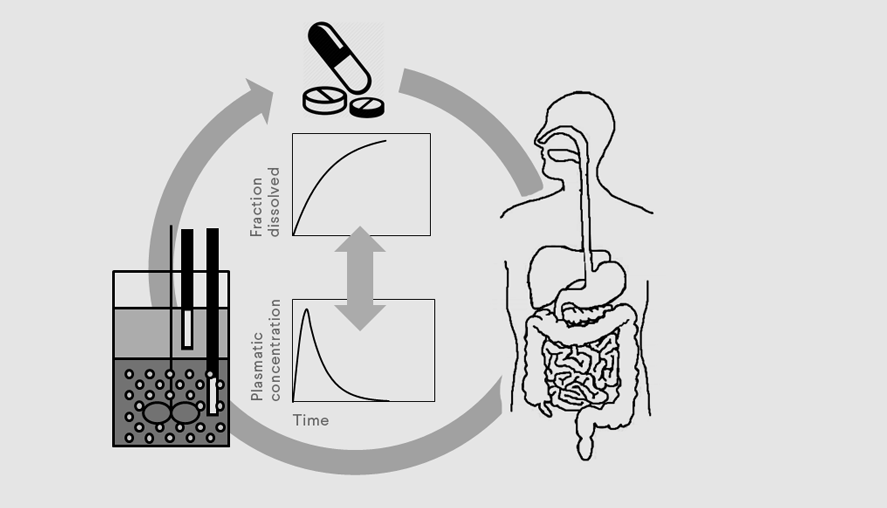
With most of the drugs under development being poorly soluble compounds, it is crucial to understand drug behavior in vivo in order to develop pharmaceutical formulations that allow optimal uptake of the drug. Novel formulations also trigger a need for appropriate methodology for in vitro testing. In this project, we combine different techniques such as kinetic measurements under biorelevant conditions, methods for solid state and physicochemical characterization, and mathematical modelling to fully understand the behavior of a drug in vivo. The identified distributional pathway in human gastrointestinal tract than serves as a rationale for formulation development as well as for the development of the methodology for in vitro testing. We are working towards a design of appropriate dissolution methodology to predict drug performance in vivo considering critical phenomena that might affect bioavailability of the drug of interest. These can involve pH induced precipitation of weak basic compounds in the human intestine, phase separation of supersaturating formulations, intestinal stability of prodrugs or suboptimal behavior of the drug in presence of an absorptive compartment.
Understanding the cellular uptake of nanoparticles
Drug absorption from gut is an important step which precedes successful treatment by orally administered drugs. The drug is absorbed mostly as a dissolved molecule, however, not much is known about the absorption of solid particles, e.g. nanocrystals. Transport of solid particles through the intestinal wall can be studied in vitro, using cell cultures. The biggest issue lies in the discernment of dissolved molecules from solid particles. For this purpose, a methodology based on the spectral or physico-chemical differences of these two forms needs to be developed and optimized.
Unraveling relationship between surface energy and dissolution rate of co-milled materials
The work focuses study of process-induced disorder of particulate solid surfaces, formed e.g. by milling or high-shear mixing and characterization of surface property modifications of studied materials. Furthermore, the effect of surface heterogeneity on the interaction strength between two particulate solids and the effect of this interaction on the dissolution of prepared mixtures is studied.
-
Preclinical in-vivo testing
Factors affecting drug absorption
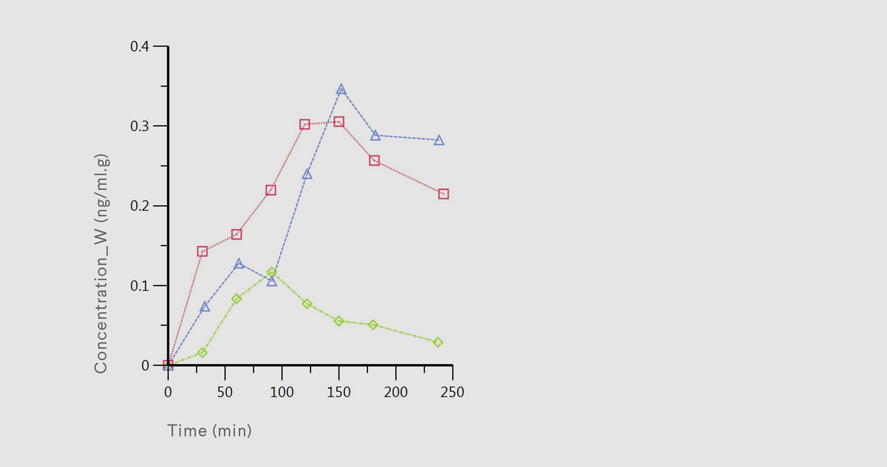
The aim of this project is to study the influence of various factors on gastrointestinal absorption of selected drugs. We especially focus on influence of gastric pH and on the food effect and on determination of pharmacokinetic characteristics of various formulation modifications. We develop appropriate in-vivo rat models, where we monitor serum drug concentration-time profiles. Broad aim of this research area is to predict formulation-specific pharmacokinetic properties in men based on preclinical data.
Lymphatic absorption of orally administered drugs
Due to the unique anatomy and physiology, there is potential to use lymphatic system as a drug delivery tool. Lymphatic drug transport has certain advantages, such as eliminating the first-pass effect, targeting drugs to specific areas of lymphatic circulation, or controlling the rate of drug delivery to systemic circulation. The aim of this project is to develop rat model for in-vivo testing of lymphatic absorption.